Periodic table block
A block of the periodic table is a set of groups of chemical elements whose valence electrons occupy, in the ground state, orbitals that share the same azimuthal quantum number ℓ, i.e. belonging to the same sub-electronic layers. As a block in the periodic table, chemical elements are grouped according to the most energetic atomic orbitals of their electron shell. A block combines several groups of the periodic table.
The periodic table block names (s, p, d, f) originated from descriptions of spectroscopic lines of atomic orbitals: sharp, principal, diffuse, and fundamental. No g-block elements have been observed to date, but the letter was chosen because it is next in alphabetical order after f.
These sublayers being designated by the letters s, p, d, f or even g, the corresponding blocks are designated by these same letters. There are four blocks in the standard periodic table, plus a fifth appearing from the hypothetical 8th period:
block s for ℓ = 0;
block p for ℓ = 1;
block d for ℓ = 2;
block f for ℓ = 3;
block g for ℓ = 4 in extended periodic tables.
This term was probably introduced in 1928 by Charles Janet.
If the periodic table block cover a certain physical reality for the first periods of the periodic table, their contours become much more blurred from the 6th period. Thus, the boundary between block d and block f is not consensual among chemists: lanthanum and actinium are alternately placed in either block by a roughly equivalent number of sources, which also leads to placing lutetium and lawrencium alternately in either block as well.
Block s
This block includes hydrogen, helium and the elements of the 1st and 2nd main group (alkali metals and alkaline earth metals).
The s block is on the left edge of the periodic table and includes all the chemical elements of group 1 and group 2. Helium, located at the top right of the table, is also part of the s block due to its electronic configuration. which, like that of hydrogen, contains only electrons from an s subshell. Helium is thus an exception in the periodic table, because it belongs to a block which is not that of its group, in this case group 18.
Apart from the elements of the 1st period, the elements of the s block are very reactive metals belonging to the families of alkali metals and alkaline earth metals. During the 1st period, hydrogen is a very reactive non-metal, while helium is a noble, chemically inert gas. All these elements are characterized by the fact that the highest energy subshell of their valence electrons in the ground state is of type s, corresponding to the azimuthal quantum number ℓ = 0.
Block p
All other main group elements belong to this block, i.e. the earth metals, carbon group, nitrogen group, chalcogens, halogens and noble gases.
The p block is located on the right edge of the periodic table and contains the chemical elements of the 13th, 14th, 15th, 16th, 17th and 18th groups of the table, except helium, which belongs to the s block.
Block p contains the greatest diversity of elements. It is the only one that contains both solid, liquid (bromine) and gaseous elements in the standard state. It is also the only one that contains both metals, metalloids and non-metals. All these elements are characterized by the fact that the highest energy subshell of their valence electrons in the ground state is of type p, corresponding to the azimuthal quantum number ℓ = 1.
Block d
These electrons are not in the outermost shell , but one below (cf. electron configuration , structural principle ). Since these elements only have one or (mostly) two outer electrons each , they show a similar chemical behavior. All subgroup elements belong to this block.
Block d is located in the center of the periodic table and contains all the chemical elements of the 3rd, 4th, 5th, 6th, 7th, 8th, 9th, 10th, 11th and 12th groups of the table.
All the elements of block d are metals and all those groups 4 to 11 are transition elements. Scandium and yttrium in group 3 and copernicium in group 12 are also, but lutetium and lawrencium in group 3 are lanthanide and actinide, respectively, while zinc, cadmium and mercury in group 12 belong to another type of metals sometimes called poor metals or post-transition metals (neither of these terms is widely used).
All these elements are characterized by the fact that the highest energy subshell of their valence electrons in the ground state must, by application of the Klechkowski rule, be of type d, corresponding to the azimuthal quantum number ℓ = 2. Lawrencium is an exception here, because its ground state electronic configuration is [Rn] 7s2 5f14 7p1 and therefore does not contain an electron in a d subshell.
Block f
These electrons are not in the outermost shell either, but in the third outermost shell. The chemical similarity in this block is therefore even more pronounced than in the d-block. The f-block includes the lanthanoids and actinides.
The block f is usually represented under the periodic table in 18 column format or in the left center of the table in 32 column format. The elements that constitute it do not belong to any of the 18 groups in the table and are located between group 2 and group 3.
All the elements of block f are, over the 6th period, lanthanides and, over the 7th period, actinides. These are sometimes called internal transition metals. The chemistry of lanthanides is very similar to that of Group 3 elements, so the whole is collectively referred to as rare earths.
All these elements are characterized by the fact that the highest energy subshell of their valence electrons in the ground state must, by application of the Klechkowski rule, be of type f, corresponding to the azimuthal quantum number ℓ = 3. Lanthanum, actinium and thorium are exceptions, because their electron configuration in the ground state is respectively [Xe] 6s2 5d1, [Rn] 7s2 6d1 and [Rn] 7s2 6d2, and therefore does not contain d ‘electron in an f subshell.
Block g
Block g is hypothetical and is only represented in periodic tables extended beyond the 7th period. The g-block is a segment of the superactinoids.
Periodic Table of Elements | Complete List of Chemical Elements by Group, Name, Symbol, Color and Type
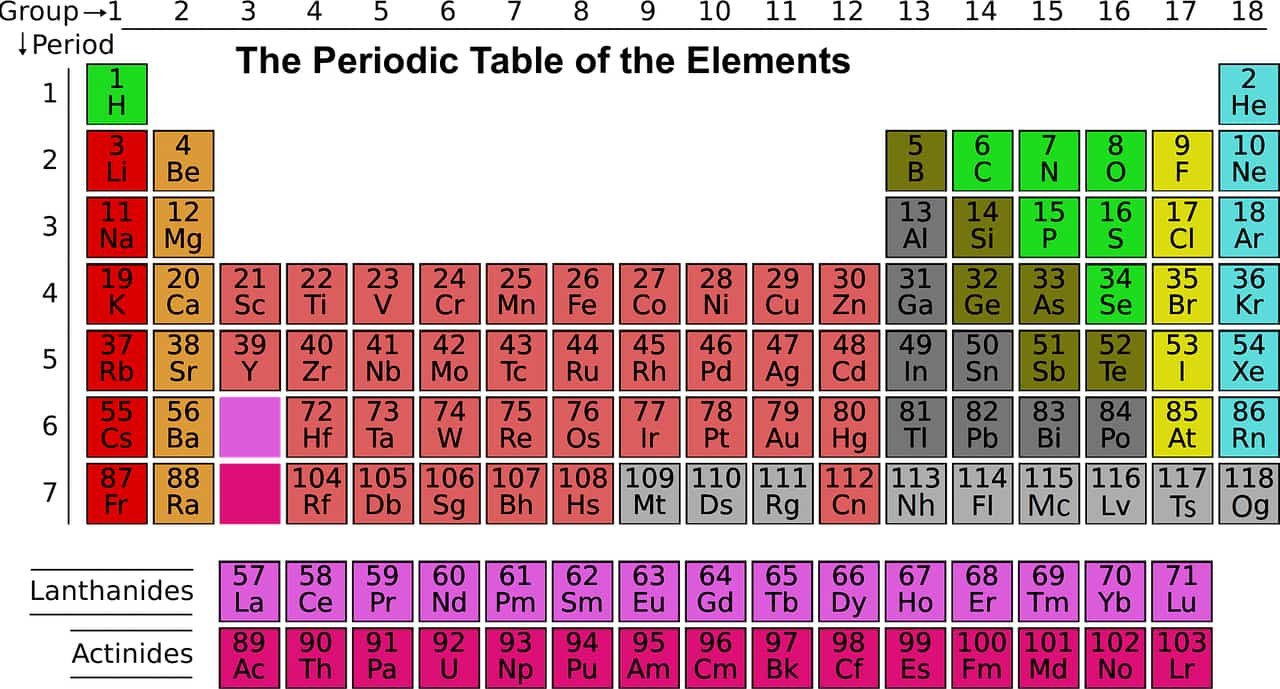
Photo credit: Wikimedia Commons


