Ionizing Radiation
This article discusses the radiation of light rays alpha (α), beta (β), gamma (γ), radiation and neutrons. Certain types of ionizing radiations have sufficient energy to rip electrons out of their orbits around atoms and disrupt the balance between electrons and protons, which has the effect of positively charging atoms. Electrically charged molecules and atoms are called ions. Any radiation that can produce ions is ionizing radiation. The following types of ionizing radiation:
Alpha radiation (α)
Alpha radiation, emitted by radioactive atoms, is a collection of helium nuclei consisting of two protons and two neutrons.
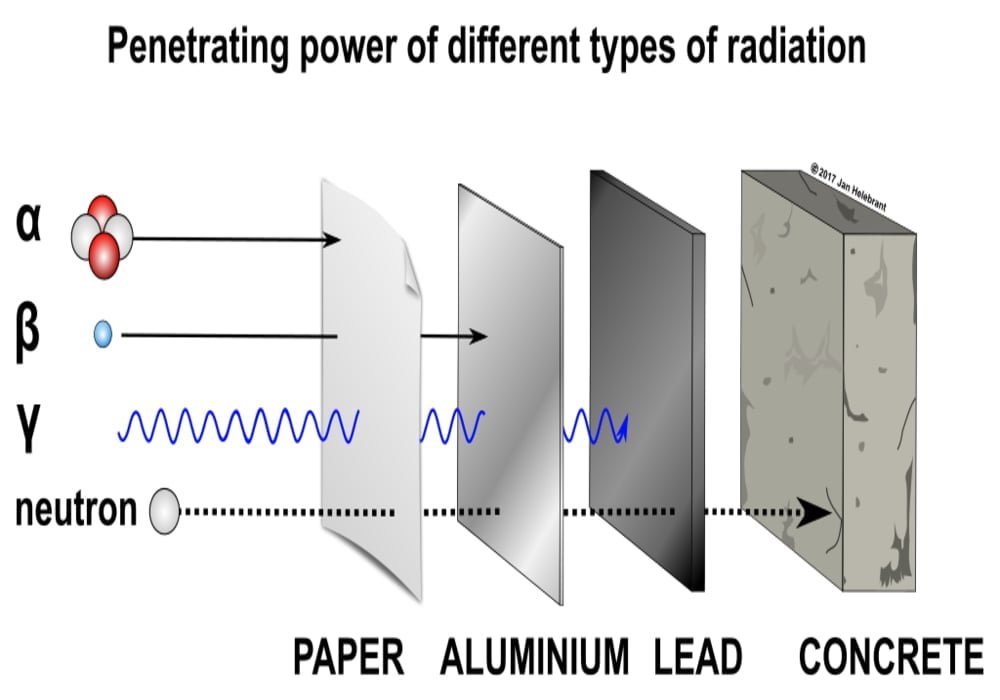
How to protect yourself from it? Heavy and electrically charged, the helium nucleus is quickly and easily stopped by the electromagnetic field and the atoms that make up matter. Therefore they were stopped by a piece of paper.
Beta radiation (β)
Beta radiation, emitted by radioactive atoms, is a beam of electrons. Beta radiation causes more damage than alpha radiation because it is electrically charged.
How to protect yourself from it? To protect against beta radiation, a simple sheet of aluminum of a few millimeters will suffice. You can also use a sheet of glass or a one-centimeter plexiglass screen, which will stop most beta particles.
Gamma radiation (γ)
Gamma radiation consists of high energy photons. This radiation will penetrate more into the body than alpha and beta radiation, but change the fewer particles it encounters.
How to protect yourself from it? Armor must be very thick. For example, to reduce this radiation by only 30%, it is necessary to be behind 6 cm of lead, 30 cm of concrete or 54 cm of soil.
X radiation (x rays)
X-rays are made up of photons (particle components of light). This radiation is used to observe through material (baggage screening at airports, x-rays for example).
How to protect yourself from it? The strong thickness of concrete or lead protects it.
Neutron radiation
Neutron radiation occurs when neutrons are ejected from the nucleus by nuclear fission and other processes. A nuclear chain reaction is an example of nuclear fission, in which a neutron released from one fission atom causes another atom to undergo fission, releasing more neutrons. Unlike other radiation, neutron radiation is absorbed by materials that have many hydrogen atoms, such as paraffins and plastics.
Read also: Radiation | Classification and Type: Electromagnetic, Ionizing and Non-ionizing, Particle
Radioactivity and its effects
What is ionizing radiation? How to protect yourself from it?
In our daily lives, we are surrounded by many types of radiation – or rays –, visible or invisible. But most of the radiation in our daily lives – radio, cell phones, microwaves – is not ionized.
Radiation is the emission of energy and/or a beam of particles.
Some radiations (X and gamma) are said to be ionizing because they emit rays with sufficient energy to convert the atoms they pass through into ions (atoms that have lost or gained one or more electrons). This can make the material unstable.
An atom – unstable by nature or after contact with radiation – attempts to stabilize itself by emitting different types of radiation:
with loss of protons and neutrons: alpha radiation;
by converting a neutron into a proton or vice versa: beta minus or beta plus radiation;
by emitting photons (particles that make up light): X and gamma rays.
Radiation – commonly called “rays” – causes different effects on the body depending on the type of radiation and the dose received.
The energy released is actually not the same for all types of radiation, and therefore the ways to protect yourself against it are different. For example, one sheet of paper is enough to stop alpha radiation, but it takes a meter of concrete or lead to stop gamma radiation.
What is the Source of Ionizing Radiation?
Sources of ionizing radiation include radioactive materials and radiation-generating machines.
Radioactive materials can occur naturally (such as the uranium and radium found on earth) or man-made in accelerators or reactors.
Radiation-generating machines, such as medical X-ray machines, produce ionizing radiation electronically and stop producing radiation when turned off. Equipment containing radioactive material, such as some industrial radiography equipment, cannot be turned off because the radioactive source emits ionizing radiation. These sources must be protected (that is, surrounded by radiation-blocking material) to prevent or reduce radiation exposure.
What are the Types of Ionizing Radiation?
The five types of ionizing radiation—alpha particles, beta particles, positrons, gamma rays, and X-rays—are the main focus of this Ionizing Radiation Safety and Health Topic page.
This page also introduces other types of ionizing radiation, particle neutron cycles, although a significant worker dose of neutrons is likely near the reactor or when using a neutron source (e.g., californium (Cf)-252, americium (Am)-241/beryllium (Be), plutonium (Pu)Be). Worker doses of neutrons can also occur in certain radiological emergencies.
Exposure to ionizing radiation
We are constantly exposed to natural and artificial sources of radioactivity. The IRSN regularly assesses the exposure of the population, patients and workers exposed to ionizing radiation, in order to monitor their changes and propose to public authorities the necessary measures for their control.
Two types of exposure to ionizing radiation:
We are talking about irradiation for external exposure to ionizing radiation, that is, when a person is exposed from the outside to ionizing radiation emitted by radioactive sources located in the vicinity. In this case, exposure ceases as soon as the source of radioactivity is removed from the person or if a screen (shield) is installed between the person and the source.
We are talking about contamination for internal exposure to radioactive particles, that is, when radioactive elements have entered the body. This can occur by inhaling radioactive particles in the air, by ingesting food contaminated with radioactive particles, or by direct contact with skin or wounds (we are talking about “external contamination”). During contamination, exposure to radioactive particles continues as long as the source is in or in contact with the body.
How to measure radioactivity?
There are 3 main units of measurement for radioactivity: Becquerel (Bq), Gray (Gy) and Sievert (Sv).
Becquerel is the international unit for measuring radionuclide activity; it corresponds to the number of disintegrations per second. The radioactivity of a substance is defined as the amount of Bqs in a given amount of matter. The natural activity of an adult’s body is 70 kg, for example 10,000 Bq, one liter of sea water is 12 Bq and one kg of granite is 7,000 Bq.
Gray is primarily used in radiotherapy to measure the amount of energy given off by radiation for every kg of tissue it passes through. We speak of the “absorbed dose” to represent the energy transmitted by a radioactive atom to the part of the body that it comes into contact with.
The sievert or its sub-multiple, millisievert (mSv = 0.001 Sv), is a unit of measurement used in radiation protection to measure the effects of radiation on living organisms. It takes into account the type of radiation, the dose received by the body and the sensitivity of the tissue through which it passes.
This unit is most often used for evaluation of health risks because it allows comparison of the effects of the same dose exerted by radiation of a different nature on organisms, organs or tissues that do not have the same sensitivity to radiation.
Interaction with other materials
Of the three common types of radiation emitted by radioactive materials, alpha, beta, and gamma, beta has moderate penetrating power and moderate ionizing power. Although beta particles released by different radioactive materials vary in energy, most beta particles can be stopped by a few millimeters of aluminum. However, this does not mean that beta-emitting isotopes can be completely shielded by such a thin shield: because they slow down in matter, beta electrons emit secondary gamma rays, which are more penetrating than beta per se. Shields made up of lower atomic weight materials produce lower energy gamma, making such shields somewhat more effective per unit mass than those made of high Z materials such as lead.
Composed of charged particles, beta radiation is more strongly ionizing than gamma radiation. As they pass through matter, beta particles are slowed down by electromagnetic interactions and can emit bremsstrahlung x-rays.
In water, beta radiation from many products of nuclear fission typically exceeds the speed of light in that material (i.e. 75% of light in a vacuum), and thus produces blue Cherenkov radiation as it passes through water. The intense beta radiation from the pool reactor fuel rods can thus be visualized through the transparent water covering and protecting the reactor.
Health effects
Radiation damages tissues and / or organs depending on the dose received or absorbed, which is expressed in a unit called the gray (Gy). The damage that can result from an absorbed dose depends on the type of radiation and the sensitivity of different tissues and organs to this radiation.
The effective dose is used to measure ionizing radiation in terms of harmfulness. The Sievert (Sv) is the unit of effective dose that takes into account the type of radiation and the sensitivity of tissues and organs.
This is a very large unit, so it is more convenient to use smaller units such as millisievert (mSv) or microsievert (μSv). There are 1000 μSv in an mSv and 1000 mSv in an Sv. In addition to the amount of radiation (dose), it is also often useful to indicate the rate at which the dose is delivered (dose rate), in μSv / hour or in mSv / year, for example.
Beyond certain thresholds, radiation can alter the functioning of tissues and / or organs and produce acute effects such as redness of the skin, hair loss, radiological burns or acute radiation syndrome. These effects become more severe as the dose and dose rate increase. For example, the threshold dose for the onset of acute radiation syndrome is approximately 1 Sv (1000 mSv).
If the dose is low and / or diffused over a long period (low dose rate), the risk is considerably lower because the likelihood of lesion repair is greater. But there is still a risk of long-term effects like cancer, which can appear years or even decades later.
Effects of this type do not always occur, but their likelihood is proportional to the dose. The risk is greater for children and adolescents because they are significantly more sensitive to radiation exposure than adults.
Epidemiological studies carried out on irradiated populations (survivors of an atomic bombardment or patients treated with radiotherapy, for example) have shown a significant increase in the risk of cancer for doses above 100 mSv. More recently, epidemiological studies in subjects exposed in a medical setting during their childhood (pediatric computed tomography) seemed to indicate that the risk of cancer could increase even at lower doses (between 50 and 100 mSv).
Prenatal exposure to ionizing radiation can induce brain damage in the fetus when the fetus receives an acute dose greater than 100 mSv between 8 and 15 weeks gestation or greater than 200 mSv between 16 and 25 weeks gestation.
Before the 8th week and after the 25th week of pregnancy, studies in men have shown no radiological risk for the brain development of the fetus. Epidemiological studies indicate that the risk of cancer following fetal radiation exposure is similar to that resulting from exposure in infancy.
Summary of alpha (α), beta (β), gamma (γ) radiations
Alpha radioactivity: Alpha radioactivity is radiation caused by alpha decay which is radioactive decay where an atomic nucleus emits an alpha particle which turns into another nucleus whose mass number is reduced from 4 and the atomic number of 2 becomes the cause of the loss of alpha particles i.e. analoging with a helium nucleus4.
Beta radioactivity: Beta radioactivity is a type of radioactive decay in which beta particles (electrons or positrons) are emitted. We’re talking about beta+ radioactivity when a positron is emitted, but we’re talking about radioactivity – when an electron is emitted.
Gamma radioactivity: Gamma radioactivity is radiation caused by gamma decay. Most often, this decay accompanies alpha or beta decay. Indeed, when emitting alpha or beta rays, the nucleus becomes excited. When emitting electromagnetic gamma radiation, the nucleus can fall back into a more stable state.
Resources: PinterPandai, U.S. Environmental Protection Agency, Health Physics Society, Ministry of the Environment Government of Japan, Occupational Safety & Health Administration
Photo credit: Openclipart, Juhele / Wikimedia Commons
Photo explanations: penetrating power of various types of radiation – alpha, beta, gamma, and neutrons.