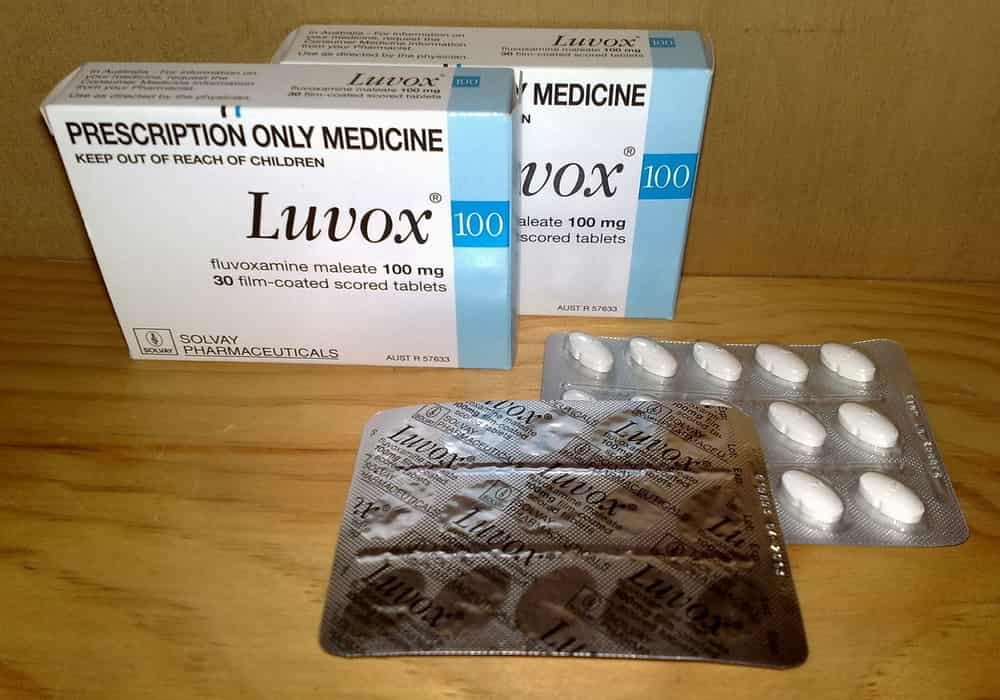
Fluvoxamine
Fluvoxamine is an anti-depressant that increases the availability of serotonin – a chemical messenger – in the brain. Low serotonin levels are indeed associated with depressive episodes. This medication belongs to the class of selective serotonin reuptake inhibitors.
Its side effects are: constipation, headache, anxiety, irritation, sexual problems, dry mouth, sleep problems and a risk of suicide at the start of treatment by lifting the psychomotor inhibition. But they seem much lower than with other SSRIs (see below).
Drugs, including alcohol, coffee and tobacco, should not be consumed while taking this medication and its prescription is not recommended for pregnant women and young people under the age of eighteen.
It can also cause manic attacks leading to aggressive, violent behavior. The dosage should be prescribed by a doctor.
In the event that there are sexual dysfunctions (hypoactive sexual desire, anorgasmia, arousal disorder, etc.) prior to the onset of a depressive state, the use of fluvoxamine should imperatively be avoided.
Its multiplier effect of the effect of caffeine on the human body would be around 5 and could cause serious disorders up to cardiac arrest, since it is a potent inhibitor of cytochrome P450-1A2 which oxidizes caffeine. to make it inactive. Fluvoxamine also inhibits the following CYP450 subunits: 2C9, 2D6, 2B6, 2C19 and 3A4. So there are many interactions with drugs. Cautiously use drugs such as diazepam, clonazepam, NSAIDs and some anti-diabetics with this one. Combinations with olanzapine, imipramine, haloperidol, clomipramine and tamoxifen are strongly contraindicated (increase by a factor of 2 to 4 in their plasma concentrations).
Fluvoxamine may have a (very modest) sedative effect due to the presence of the halogen compound trifluoro on the aromatic ring.
COVID-19: Trial of antidepressant fluvoxamine after promising results
Researchers at the Research Institute of the McGill University Health Center (RI-MUHC) in Quebec launch a randomized placebo-controlled trial to confirm the effectiveness of the antidepressant fluvoxamine (Luvox, Floxyfral) in slowing the progression of symptoms of COVID-19.
The drug, a statement from the researchers reports, has already been shown to be effective in reducing lung damage in animal studies and in a small, placebo-controlled human trial.
Drs. Todd Lee and Emily McDonald, RI-MUHC and McGill University, are launching the Canadian component of the STOP COVID2 clinical trial, currently underway in the United States.
This study follows the STOP COVID trial, whose results published in November 2020 in the Journal of the American Medical Association (JAMA) showed that fluvoxamine was associated with reduced clinical deterioration in people with COVID- 19.
In the STOP COVID trial, led by Eric Lenze and Angela M. Reiersen of the University of Washington in St. Louis and their colleagues, “0 of 80 patients assigned to the fluvoxamine group and 6 of 72 patients assigned to the placebo group, have suffered clinical deterioration, ”says Dr. McDonald. “This is a statistically significant difference. We now hope to corroborate these results with a larger sample. ”
“Fluvoxamine is a safe, inexpensive drug approved by Health Canada for the treatment of depression and obsessive-compulsive disorder,” the release reported.
“Researchers will determine if it reduces the risk of developing severe shortness of breath, requiring oxygen, and being hospitalized due to COVID-19, and will see if it decreases persistent symptoms”.
Read also: Oximeter | How does it work? What is Normal Range of Blood Oxygen Level?
“Based on the promising results of the previous clinical trial, we are optimistic that this drug can prevent hospitalization and reduce the strain on the healthcare system,” says Dr. Lee.
“Participants will receive a safe dosage of fluvoxamine (or a placebo) at home, along with a thermometer and oxygen saturation meter, which they can keep”.
The trial is open to non-hospitalized adults who have tested positive for SARS-CoV-2, who have had symptoms of COVID-19 for up to 6 days, and who have a risk factor for clinical deterioration. related either to their age (40 years or older), to their race or ethnicity (African-Canadian, Hispanic or indigenous) or to a medical condition (such as asthma, high blood pressure, overweight or diabetes). Registration is done at stopcovid2.idtrials.com.
Sources: Washington University School of Medicine in St. Louis, Clinicaltrials.gov, Science News, CBS News
Photo credit: Wikimedia Commons