Radiation Types
Radiation is energy that is emitted from a source and propagates through space in the form of waves or particles. There are different radiation types, each with its own characteristics or properties.
Find out about the different types of radiation, how they interact with atoms, and how they can affect you.
Radiation is a type of energy that can propagate through space in the form of waves (electromagnetic radiation) or particles moving at high speeds (particle radiation).
You have been exposed to various forms of radiation throughout your life, perhaps without knowing it!
Radiation Types
Types of radiations are: Electromagnetic, Ionizing and Non-ionizing, Particle.
1. Electromagnetic radiation
Electromagnetic radiation consists of waves. These waves contain electrical and magnetic energy.
The electromagnetic spectrum covers the entire energy range, from very low energies, such as radio waves, to very high energies, such as gamma rays.
Electromagnetic radiation is characterized by frequency (number of waves per second) and wavelength (distance between adjacent wave crests). The higher the frequency, the shorter the wave. For example, gamma rays have very high frequencies and very short wavelengths. This wave also has a lot of energy!
There are seven natural forms of electromagnetic radiation:
1. Gamma rays have the highest energy and the shortest wavelength.
2. X-ray
3. Ultraviolet light
4. Visible light
5. Infrared radiation
6. Microwave
7. Radio waves that have the least energy and longest wavelength.
The only parts of the electromagnetic spectrum that our senses can detect directly are infrared (feels like heat) and visible light. We cannot see or feel radio waves, x-rays, and gamma rays, but they can pass through the body.
Electromagnetic radiation travels in tiny packets (quanta) of energy called “photons” (energy packets of zero electric charge moving through a vacuum at the speed of light, 2.998 x 108 m/s).
Read also: Physics Research Fields | Major fields of physics, with subfields, theories and concepts
2. Ionizing and non-ionizing radiation
Radiation types in the presence of ionizing and non-ionizing:
Ionizing radiation
Ionizing radiation has enough energy to eject electrons from their original atoms, releasing ions.
Far ultraviolet radiation, X-rays and gamma rays are the three forms of ionizing radiation. This type of high energy radiation can quickly cause cancer and even destroy cells instantly. This is why we were required to wear lead aprons to take dental x-rays and the technician went to a different room to use the x-ray machine.
The amount of radiation in one x-ray is harmless! But large amounts of x-ray radiation can be dangerous. This is why technicians are placed in different rooms to use the X-ray machine.
Ionizing radiation is a type of radiation that can release, or ionize, electrons from other atoms as they pass through matter. These include electromagnetic waves and subatomic particles.
Examples of ionizing radiation are:
Certain forms of electromagnetic radiation, including:
- high energy ultraviolet radiation
- X-ray
- neutron
- gamma rays (radiation)
- particle radiation, such as:
- alpha particle
- beta particles (electrons)
Sources of exposure to ionizing radiation
1. Natural sources of ionizing radiation include:
-
- cosmic (from outer space)
- from rocks and soil
- emitted by these sources is called “background radiation”.
2. Man-made sources of ionizing radiation include:
-
- nuclear energy
- medical devices, such as:
- x-ray machine
- CT scanner
- security devices, such as X-ray tunnels for baggage inspection
- industrial devices used for scientific research and measurement
Effects of ionizing radiation on living things
Effects on humans Depending on the intensity of the radiation and on the part of the body where it is generated, the radiation can be either harmless, or above the 250 mSv (milli-sievert) equivalent dose to produce various effects.
Symptoms in humans due to radiation accumulated on the same day (the effect is reduced if the same amount of Siévert is accumulated over a longer period):
Dosage Accepted Effect:
- 0 – 0.25 Sv Almost none
- 0.25 – 1 Sv Some people experience nausea and loss of appetite, and the bone marrow, lymph nodes, or spleen may be damaged.
- 1 – 3 Sv Mild to severe nausea, loss of appetite, infection, more severe bone marrow loss, as well as damage to lymph nodes, spleen, with possible recovery.
- 3 – 6 Sv Severe nausea, loss of appetite, bleeding, infection, diarrhea, crusting, infertility and death if left untreated.
- 6 – 10 Sv Same symptoms, more damage to the central nervous system. Possible death.
- > 10 Sv Paralysis and death.
Symptoms in humans from radiation accumulated over a year, in millieverts (1 Sv = 1000 mSv):
- 2.5 mSv: Radiation average global yearly.
- 5.5 – 10.2 mSv: Average natural values in Guarapari (Brazil) and in Ramsar (Iran). No harmful effects.
- 6.9 mSv: CT scanner.
- 50 – 250 mSv: Limits for prevention and emergency workers, respectively.
Type of ionizing radiation (table)
| Type of ionizing radiation | How it travels and penetrates | How it delivers dose to the body |
|---|---|---|
| Alpha particles (α) | Alpha particles cannot penetrate most other materials. A piece of paper, the dead outer layers of skin, or even a few inches of air are sufficient to stop alpha particles. | Radioactive material that emits alpha particles can be very harmful to living cells when alpha particles are inhaled, ingested, or absorbed into the blood stream (e.g., through a cut in or area of non-intact skin). |
| Beta particles (β) | Beta particles can travel up to several feet in the air. Beta particles can be stopped by some elements, such as: plastics, aluminum, or a block of wood. Beta particles should never be shielded with lead or other high atomic number shields, which could result in X-rays being released. | Some beta particles are capable of penetrating the skin and causing radiation damage, such as skin burns. Beta particles are most harmful to living cells when they are inhaled or ingested. |
| Gamma rays (γ) and X-rays (electromagnetic radiation) | Gamma rays and X-rays are very penetrating and can travel great distances. Lead or concrete is able to reduce the intensity of gamma rays and X-rays. | Gamma rays and X-rays can easily pass completely through the human body; however, a fraction of the energy can be absorbed by tissue and can damage living cells. |
| Neutron particles | Neutrons have an exceptional ability to penetrate materials. Hydrogen-containing materials (concrete or water) are best for shielding neutrons. | Neutrons can contribute significantly to radiation dose. |
Examples type of ionizing radiation
| Type of ionizing radiation | Examples |
|---|---|
| PARTICULATE RADIATION (sub-atomic particles with mass, such as alpha and beta particles, electrons, and neutrons) | |
| Alpha particles (α)Positively charged particles consisting of two protons and two neutrons emitted from the nucleus of some radioactive atoms. An alpha particle is the nucleus of a helium atom.Unstable atoms with a low neutron-to-proton ratio may emit alpha particles. | Radionuclides that emit alpha particles include:
For example, a Po-210 atom has 84 protons and 126 neutrons, and is unstable (i.e., radioactive). To become more stable, the Po-210 atom ejects an alpha particle, consisting of two protons and two neutrons. Having lost two protons and two neutrons, the radioactive Po-210 atom becomes stable lead-206 (Pb-206), with 82 protons and 124 neutrons. |
| Beta particles (β-) and Positrons (β+)Beta particles (β-)Negatively-charged, fast-moving electrons emitted from the nucleus of various radionuclides. Unstable atoms with a high neutron-to-proton ratio emit negatively-charged beta particles.
Positrons (β+) Positively-charged, fast-moving electrons emitted from the nucleus of certain radionuclides. Unstable atoms with a low neutron-to-proton ratio can emit positrons. |
Beta particles (β-)Some radionuclides that emit beta particles include:
For example, a carbon-14 atom has six protons and eight neutrons, and is unstable (i.e., radioactive). To become more stable, the C-14 atom releases radiation by turning a neutron into a proton and ejecting an electron (i.e., a beta particle). Having gained a proton and lost a neutron, the radioactive C-14 atom becomes stable nitrogen-14 (N-14), with seven protons and 7 neutrons. Positrons (β+) Fluorine-18 (F-18) is an example of a positron-emitting radionuclide that is commonly used in medical facilities for positron emission tomography (PET) scanning. An F-18 atom has nine protons and nine neutrons, and is unstable (i.e., radioactive). To become more stable, the F-18 atom releases radiation by turning a proton into a neutron and ejecting a positron. Having gained a neutron and lost a proton, the radioactive F-18 atom becomes stable oxygen-18 (O-18), with eight protons and 10 neutrons. |
| Neutron particlesNeutral (i.e., having no electric charge) particles that can be emitted from the nuclei of various unstable radionuclides. Neutrons are high-speed nuclear particles that are the only type of ionizing radiation that can make objects radioactive. | Nuclear fission and fusion reactions, as well as neutron sources (e.g., Cf-252, AmBe), neutron generators, and some particle accelerators, produce neutrons. For example, neutrons would be produced from the detonation of a fissile nuclear weapon, such as an improvised nuclear device (IND). Visit OSHA’s Radiation Emergency Preparedness and Response page for more information. |
| ELECTROMAGNETIC RADIATION (Gamma rays and X-rays) has no mass and no charge. | |
| Gamma rays (γ)High-energy electromagnetic photons emitted from the nucleus of an unstable, excited atom. Gamma rays are pure energy and can travel great distances at high speed. | Some radionuclides that emit gamma rays include:
Gamma rays are often emitted along with alpha or beta particles during radioactive decay (e.g., Co-60, Ir-192). |
| X-raysHigh-energy electromagnetic photons emitted from outside the nucleus. The primary difference between X-rays and gamma rays is that X-rays are emitted from processes outside the nucleus, but gamma rays originate inside the nucleus. | Some radionuclides that emit X-rays include:
Machines containing an X-ray tube also electronically produce X-rays. |
Non-ionizing radiation
Non-ionizing radiation does not have sufficient energy to ionize atoms or molecules (and thus cause them to gain or lose electrons).
There are several types of non-ionizing radiation. This includes in particular near ultraviolet light, visible light, infrared radiation, microwaves and radio waves. It cannot ionize atoms, but it is not completely harmless. Microwaves are energetic enough to cook our food and ultraviolet light is enough to burn us in the sun.
Non-ionizing radiation is also a form of electromagnetic radiation. This type of radiation does not have enough energy to release electrons.
Examples of non-ionizing radiation are:
radio frequency wave
microwave
infrared
visible light
Non-ionizing radiation source
Non-ionizing radiation can be natural or artificial.
Some natural sources of non-ionizing radiation include:
Lightning
Sunlight and heat
Earth’s natural electric and magnetic fields
Sources of man-made non-ionizing radiation include common things, such as:
Tanning bed
Microwave oven
Wireless devices, such as:
WL
mobile station
Wi-Fi Equipment
radio and television broadcasting antenna
lighting products, such as:
LED light
Incandescent lamps
Compact fluorescent lamp / solid fluorescent lamp
Electrical and household cables
Handheld laser and laser pointer
Read also: Comparison Light Bulbs and Different types (advantages disadvantages)
3. Particle radiation
Particulate radiation types consist of atomic or subatomic particles, such as protons, neutrons, and electrons, that have kinetic energy (energy of moving mass).
Alpha particles and beta particles emit ionizing radiation directly because they are charged and can interact directly with atomic electrons through the Coulomb force (that is, charges of the same nature repel, whereas charges of opposite nature attract).
Alpha particles consist of two protons and two neutrons. These particles are large, slow and positively charged. Alpha particles are the same as the nuclei of helium atoms.
Beta particles are small and move fast. They can have a positive charge (positrons) or a negative charge (electrons).
Neutrons are found in the nucleus of an atom, and unlike protons and electrons, they are uncharged particles.
Neutron radiation is indirectly ionizing radiation. It consists of free neutrons that have been released from atoms.
These free neutrons can react with the nuclei of other atoms to form new isotopes, which in turn can emit radiation, such as gamma rays. Neutron radiation is said to be “indirect ionizing” because it does not ionize atoms in the same way as charged particles.
Classification of Radiation Types
Radiation types can be classified:
1. By energy-conducting elements
Electromagnetic radiation – is energy that propagates through electromagnetic waves, consisting of an oscillating electric field and a mutually perpendicular magnetic field, which propagates in a vacuum at the speed of light, which is 299,792,458 meters per second. It is characterized by its wavelength or frequency and by the different bands that make up the electromagnetic spectrum. Mentioned, among others, for example electromagnetic radiation, gamma rays, x-rays and sunlight.
Cell radiation – Energy spreads through subatomic particles such as electrons, protons and others which are formed by nuclear fission such as neutrons. Thus, it is characterized by its charge, mass, and velocity, chargeable or neutral, light or heavy, and slow or fast.
Gravitational radiation – Gravitational radiation is a prediction of the equations of general relativity. They can be emitted in regions of space where gravity is relativistic, through collapsing stars.
2. By radiation source
Solar radiation – Caused by energy emitted from the sun, resulting from reactions occurring on the surface of stars. Solar radiation is propagated by electromagnetic waves.
Cherenkov radiation – Caused when electrically charged particles, at speeds greater than the speed of light in a medium, pass through an insulating medium. The characteristic blue color of a nuclear reactor is caused by Cherenkov radiation. The name is in honor of the Soviet scientist Pavel Cherenkov, winner of the 1958 Nobel Prize in Physics.
Radioactivity – Radioactivity (or radioactivity) is the property of certain types of chemical elements to emit radiation, a naturally occurring or artificial phenomenon. Artificial radioactivity occurs when there is a nuclear transformation, through atomic union or nuclear fission. Natural radioactivity occurs through radioactive elements found in nature.
3. By the effect
Ionizing radiation – It is capable of ripping any electron from an atom if its energy is greater than the energy of its bond with the atom. Particle electrical charges such as beta and alpha are considered ionizing when they have sufficient energy to ionize atoms in their path until they lose all of their energy. Only X-rays and gamma rays are ionizing radiation that observe the spectrum of electromagnetic waves, that is, they have sufficient energy to ionize atoms.
Non-ionizing radiation – These are not capable of ionizing molecules, as they do not have enough energy to tear electrons from atoms, but they can break chemical and molecular bonds. Ultraviolet radiation is considered non-ionizing because it does not have sufficient energy to extract electrons from the main atoms that make up the human body and because its penetration is very small.
Degradation of materials by radiation – This is a physical phenomenon resulting from the effects of ionizing radiation on inert matter.
4. According to radiation type
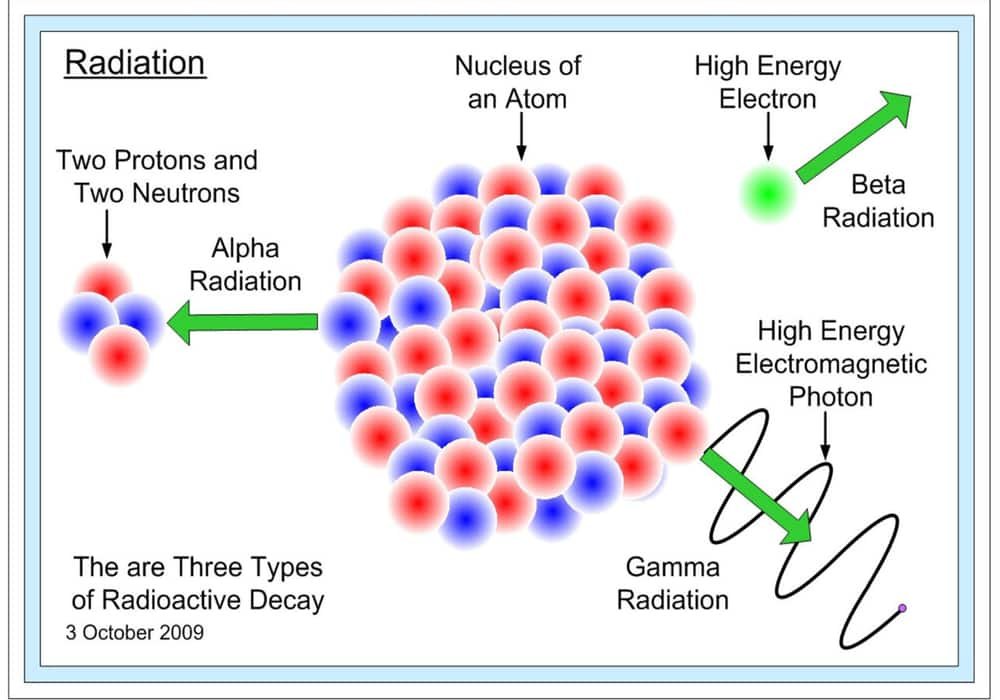
Alpha radiation (α) – Or alpha particles
It consists of two protons and two neutrons (like the nucleus of a helium atom), with a positive charge of 2e. The specified distance traveled by a particle to stop is called the “particle range”. All alpha particles in any medium and with the same energy have the same range. Because the range of alpha particles is so small, they are easily shielded. It has a low speed (20,000 kilometers per second) compared to the speed of light. Its path in the material medium is straight. Alpha particles are mainly produced in the decay of elements such as uranium, radium, plutonium, thorium, etc.
Beta radiation (β)
These are electrons that are emitted through a stable atomic nucleus. They are much more penetrating than alpha particles. Beta radiation, as it passes through the material, loses energy, and thus, ionizes atoms in its path. It has a speed of about 270,000 kilometers per second. To protect the beta particles, aluminum or plastic should be used.
Gamma radiation (γ) – Gamma radiation
Is an electromagnetic wave, and has a very large penetrating power. As they pass through substances, they collide with their molecules. It has a speed of 300,000 kilometers per second.
X Radiation: It is an electromagnetic wave that has a very short wavelength (between 1 nanometer and 5 picometer). X-rays have the same characteristics as gamma rays, only differ in their formation, while gamma rays are formed inside the nucleus of atoms, x-rays are formed outside. They are widely used in medical examinations.
Neutrons – Neutrons have no charge and do not directly produce ionization, but indirectly transfer energy to other charged particles that can produce ionization. They traverse the entire electrosphere before interacting with atomic nuclei. They are very translucent and their mass is 1,675 x 10ˉ²⁷ kg. Can be protected with water, paraffin and other hydrogen-rich materials.
Sources: PinterPandai, U.S. Environmental Protection Agency, Health Physics Society, Ministry of the Environment Government of Japan, Occupational Safety & Health Administration
Photo credit: Public Domain Pictures