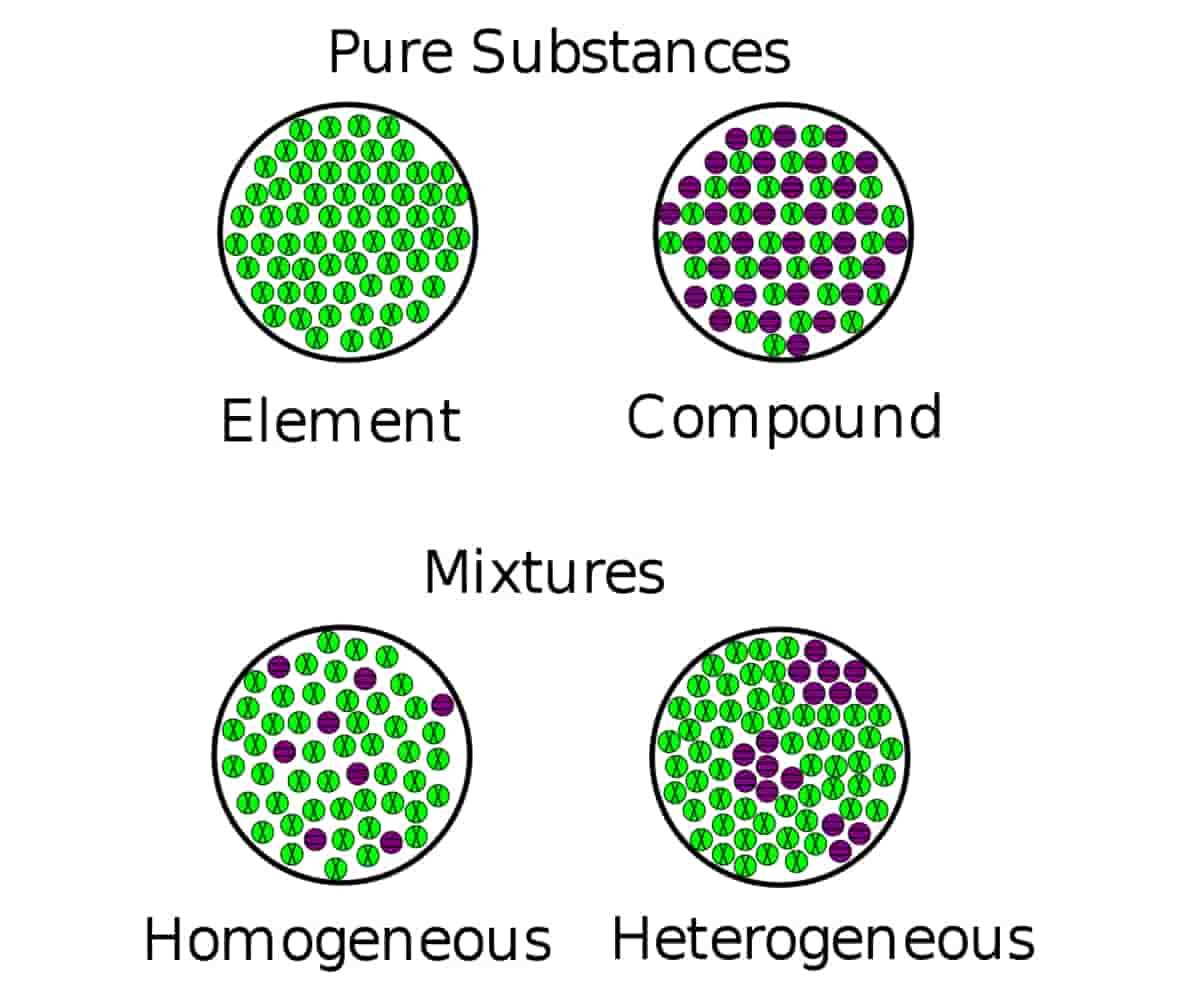
Homogeneous and Heterogeneous | Difference, Mixtures, Example
The terms heterogeneous and homogeneous refer to mixtures of materials in chemistry. The difference between heterogeneous and homogeneous mixtures lies in the degree of mixing of the materials and the uniformity of their composition.
Homogeneous and heterogeneous are two different words that we can distinguish based on the context in which we use them. The materials, mixtures, reactions, etc. can be homogeneous or heterogeneous. But the difficulty in identifying the difference between these two problems mainly arises when classifying mixtures.
Examples of homogeneous mixtures
There are several examples of homogeneous mixtures encountered on a daily basis:
- Air
- Sugar water
- Rainwater
- Vodka
- The vinegar
- Dishwasher detergent
- Steel
You cannot select the components of a homogeneous mixture or use simple mechanical means to separate them. You cannot see the individual chemicals or ingredients in this type of mixture. Only one phase of matter is present in a homogeneous mixture.
A heterogeneous mixture is a mixture in which the components of the mixture are not uniform or have localized regions with different properties. Different samples of the mixture are not identical to each other. There are always two or more phases in a heterogeneous mixture, where you can identify a region with distinct properties from another region, even if they are of the same state of matter (e.g. liquid, solid).
Examples of heterogeneous mixtures
Heterogeneous mixtures are more common than homogeneous mixtures. Examples include:
- Milk cereals
- Vegetable soup
- Pizza
- Some blood
- Gravel
- Soda ice cream
- Vinaigrette
- Mixed nuts
- Bowl of colorful candies
- Ground
Usually, it is possible to physically separate the components of a heterogeneous mixture. For example, you can centrifuge (spin) solid blood cells to separate them from blood plasma. You can remove the ice cubes from the soda. You can separate the candies according to their color.
How to distinguish homogeneous mixture and heterogeneous mixture
The difference between the two types of mixtures is a matter of scale or quantity. If you take a closer look at the sand at the beach, you can see the various components, including shells, coral, sand, and organic matter. It is a heterogeneous mixture. However, if you look at a large amount of sand from a distance, it is impossible to distinguish the different types of particles. The mixture is homogeneous. This may seem confusing!
To identify the nature of a mixture, consider its sample size. If you can see more than one phase of the material or different regions in the sample, it is heterogeneous. If the composition of the mixture appears uniform wherever you sample it, the mixture is homogeneous.
Mixtures Table (Gas, Liquid and Solid)
Colloids are heterogeneous but in appearance they seem to be homogenous because the constituent particles in the mixture are very small – 1 nanometer to 1-micrometer.
Dispersion medium (mixture phase) |
Dissolved or dispersed phase |
Solution |
Colloid |
Suspension (coarse dispersion) |
|---|---|---|---|---|
Gas |
Gas | Gas mixture: air (oxygen and other gases in nitrogen) | None | None |
| Liquid | None | Liquid aerosol:[11] fog, mist, vapor, hair sprays |
Spray | |
| Solid | None | Solid aerosol:[11] smoke, ice cloud, air particulates |
Dust | |
Liquid |
Gas | Solution: oxygen in water |
Liquid foam: whipped cream, shaving cream |
Sea foam, beer head |
| Liquid | Solution: alcoholic beverages |
Emulsion: milk, mayonnaise, hand cream |
Vinaigrette | |
| Solid | Solution: sugar in water |
Liquid sol: pigmented ink, blood |
Suspension: mud (soil particles suspended in water), chalk powder suspended in water |
|
Solid |
Gas | Solution: hydrogen in metals |
Solid foam: aerogel, styrofoam, pumice |
Foam: dry sponge |
| Liquid | Solution: amalgam (mercury in gold), hexane in paraffin wax |
Gel: agar, gelatin, silicagel, opal |
Wet sponge | |
| Solid | Solution: alloys, plasticizers in plastics |
Solid sol: cranberry glass |
Clay, silt, sand, gravel, granite |
Sources: PinterPandai, Lumen Learning, Ask Any Difference, Diffen
Photo credit: John Trombley / Wikimedia Commons (CC BY 4.0)